阿達色林
外觀
維基百科,自由的百科全書
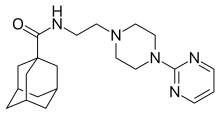 | |
| 臨床資料 | |
|---|---|
| ATC碼 |
|
| 識別資訊 | |
| |
| CAS號 | 127266-56-2 |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard(英語:CompTox Chemicals Dashboard) (EPA) | |
| 化學資訊 | |
| 化學式 | C21H31N5O |
| 摩爾質量 | 369.51 g·mol−1 |
| 3D模型(JSmol(英語:JSmol)) | |
| |
| |
阿達色林(INN:adatanserin;開發代號:WY-50,324、SEB-324)是一種混合的5-HT1A受體部分激動劑以及5-HT2A和5-HT2C受體拮抗劑。[1][2][3]惠氏公司試圖將其開發為抗抑鬱藥,但最終沒有得到推廣。[3][4]
阿達色林已被證明對缺血引起的穀氨酸興奮毒性具有神經保護作用,這種作用似乎是通過阻斷5-HT2A受體介導的。[5]
參見
[編輯]參考資料
[編輯]- ^ Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5-HT1A receptors. Neuropharmacology. April 1993, 32 (4): 331–40. PMID 8497336. S2CID 38611829. doi:10.1016/0028-3908(93)90153-T.
- ^ Kleven MS, Koek W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT2A/2C antagonist anxiolytics. I. Antipunishment effects in the pigeon. The Journal of Pharmacology and Experimental Therapeutics. February 1996, 276 (2): 388–97. PMID 8632301.
- ^ 3.0 3.1 Abou-Gharbia MA, Childers WE, Fletcher H, et al. Synthesis and SAR of adatanserin: novel adamantyl aryl- and heteroarylpiperazines with dual serotonin 5-HT(1A) and 5-HT(2) activity as potential anxiolytic and antidepressant agents. Journal of Medicinal Chemistry. December 1999, 42 (25): 5077–94. PMID 10602693. doi:10.1021/jm9806704.
- ^ Stahl, S. M. Essential psychopharmacology: neuroscientific basis and practical application
 . Cambridge, UK: Cambridge University Press. 2000: 262. ISBN 0-521-64615-4.
. Cambridge, UK: Cambridge University Press. 2000: 262. ISBN 0-521-64615-4. adatanserin.
含有內容需登入查看的頁面 (link) - ^ Dawson LA, Galandak J, Djali S. Attenuation of ischemic efflux of endogenous amino acids by the novel 5-HT(1A)/5-HT(2) receptor ligand adatanserin. Neurochemistry International. March 2002, 40 (3): 203–9. PMID 11741003. S2CID 24104458. doi:10.1016/S0197-0186(01)00082-1.
