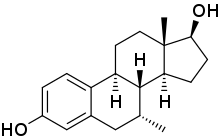
7α-甲基雌二醇
外觀
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | 7α-Methyl-E2; 7α-Me-E2; 7α-Methylestra-1,3,5(10)-triene-3,17β-diol |
| 藥物類別 | Estrogen |
| 識別資訊 | |
| |
| CAS號 | 10448-97-2 |
| PubChem CID | |
| 化學資訊 | |
| 化學式 | C19H26O2 |
| 摩爾質量 | 286.42 g·mol−1 |
| 3D模型(JSmol) | |
| 熔點 | 120 °C(248 °F) [1] |
| |
| |
7α-甲基雌二醇(英語:7α-Methylestradiol,縮寫7α-Me-E2,也稱為7α-甲基雌甾-1,3,5(10)-三烯-3,17β-二醇,7α-methylestra-1,3,5(10)-triene-3,17β-diol)是一種人工合成的甾體類雌激素藥物,對雌激素受體的親和力與雌二醇相當[2]。7α-甲基雌二醇同時也是雄激素/同化類固醇曲托龍的活性代謝產物,是曲托龍造成的雌激素效應的原因[3][4]。
另見
[編輯]- 阿美雌酮(7α-甲基雌酮)
參考文獻
[編輯]- ^ Steroid with high estrogenic effect(法文). 1965. FR 1418540.
- ^ Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. Receptor Binding as a Tool in the Development of New Bioactive Steroids. Drug Design. 1979: 169–214 [2021-06-27]. ISBN 9780120603084. doi:10.1016/B978-0-12-060308-4.50010-X. (原始內容存檔於2020-06-04).
- ^ García-Becerra R, Ordaz-Rosado D, Noé G, Chávez B, Cooney AJ, Larrea F. Comparison of 7α-methyl-19-nortestosterone effectiveness alone or combined with progestins on androgen receptor mediated-transactivation. Reproduction. 2012, 143 (2): 211–9. PMID 22065861. doi:10.1530/REP-11-0171
 .
.
- ^ Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR. Dimethandrolone (7,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase. The Journal of Steroid Biochemistry and Molecular Biology. June 2008, 110 (3–5): 214–22. PMC 2575079
 . PMID 18555683. doi:10.1016/j.jsbmb.2007.11.009.
. PMID 18555683. doi:10.1016/j.jsbmb.2007.11.009.
